
Michael C. Pirrung, Ph.D.
Department of Chemistry, UC Riverside
CURRENT GRANT ABSTRACTS, RELATED PUBLICATIONS, AND FIGURES:
Department of Defense Post-traumatic Stress Disorder Traumatic Brain Injury - Small Molecule Activators of the TRK Receptors for Neuroprotection
Traumatic brain injury (TBI) is one of the major causes of mortality and morbidity among the active Iraqi-war military. It is also a major cause of morbidity and death in children and young adults. Loss of hippocampal neurons is a common sequela to a single traumatic brain insult. This loss can occur over a period of many days following the insult, yet despite improvements in surgical treatment of the primary insult, there are currently no therapies that provide neuroprotection to mitigate this secondary or delayed damage. Thus, even moderate brain injury is associated with poor prognosis and chronic cognitive impairment. We have an ongoing research project focused on developing neuroprotective drugs that target the nerve growth factor receptor TrkA that is based on the fungal natural product demethylasterriquinone B1. Neuroprotection and the development of therapeutic agents that can prevent neuronal injury is one of the identified research gaps in TBI. Therefore, this proposal aims to develop drugs targeting the neurotrophin receptors TrkB and TrkC. There is good reason to believe that neurotrophins may be protective in brain injury.
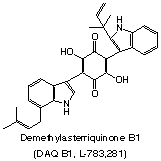
This project is being performed in collaboration with Prof. Nicholas J. G. Webster of the UC-San Diego Medical School.
Bo Lin, Michael C. Pirrung, Liu Deng, Zhitao Li, Yufa Liu, and Nicholas J. G. Webster, “Neuroprotection by Small Molecule Activators of the NGF Receptor,” J. Pharmacol. Exp. Ther., 322, 59 (2007).
University of California Cancer Research Coordinating Committee - Synthesis and biology of syrbactin natural proteasome inhibitors
This project will prepare libraries of macrolactams based on the natural products syringolin A and glidobactin A (the syrbactins).The objective of this work is to discover novel, potent, and selective macrolactam inhibitors of the mammalian proteasome with anti-cancer activity, and to develop structure-reactivity relationships for the reaction of the macrolactams with the proteasome.
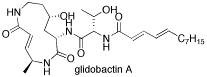
This project is being performed in collaboration with Prof. André S. Bachmann of the Cancer Center of Hawaii and the University of Hawaii-Manoa.
Tannya R. Ibarra-Rivera, John Opoku-Ansah, Sudhakar Ambadi, André S. Bachmann, and Michael C. Pirrung, “Synthesis and Cytotoxicity of Syringolin B-Based Proteasome Inhibitors,” Tetrahedron, 67, 9950 (2011).
NSF - Scope and Aplications of Amide Ligation of Hydroxyamines
This project is aimed at developing for practical peptide chemistry N-carboxyanhydride (NCA) acylations and further investigating the recently discovered amide ligation of hydroxyamines (serine) with mildly activated esters. It will elucidate structure-reactivity relationships to define the preparative utility of the ligation. The similarity of this process (with serine) to native chemical ligation (via cysteine) further suggests its use in segment-based peptide synthesis, where the segments will be generated using solid-phase peptide synthesis or NCA chemistry. Because serine ligations are performed at high concentrations and can be readily scaled, whereas native chemical ligation is performed in dilute aqueous media and is not, these methods are especially applicable to preparative syntheses. Enabling segment couplings of peptides at N-terminal serines gives our method specific relevance to serine-containing epitopes, such as oxazole natural products derived biosynthetically from serine peptides, and the glycoprotein sequon. The utility of serine ligations in these contexts will be demonstrated with the synthesis of a glycoprotein fragment, two oxazole natural product fragments, and the neuropeptide gonadotropin-releasing hormone (GnRH) and its therapeutic derivative leuprolide.
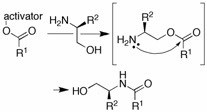
The surprising observation was made that weakly activated esters follow an initial trans-esterification pathway in the formation of hydroxy amides. This permits a significant broadening of the specialized native chemical ligation method (with cysteine) to large segment peptide syntheses.
Michael C. Pirrung, Fa Zhang, Sudhakar Ambadi, and Tannya R. Ibarra-Rivera, “Reactive Esters in Amide Ligation with b-Hydroxyamines,” Eur. J. Org. Chem., 4283 (2012).
NIH NIAID - Sustainable Synthesis of Enfurvitide
The objective of this application is the development, using novel peptide coupling/ligation chemistries, of two new syntheses of enfuvirtide that are far more sustainable than the extant enfuvirtide synthesis.




